According to infectious disease specialists, the Ministry of Health is putting up barriers to access to HIV drugs

A circular from the Ministry of Health has raised concerns among infectious disease specialists and experts about the barriers it could impose on HIV treatment in Colombia. This is external circular 006, sent by the Minister of Health on February 24 to EPS, health care providers, doctors and other actors.
In the document, the Ministry of Health establishes new processes for prescribing antiretroviral treatments for HIV, which are part of the Basic Health Plan (PBS) in Colombia. Now, when a health professional wants to order this treatment, they must do so through Mipres, a tool for prescribing health technologies not funded with UPC resources.

Ministry of Health Circular. Photo: Ministry of Health
According to the health portfolio, the measure is taken with the purpose of monitoring and evaluating access to health technologies in the provision of health services. “It is necessary to allow the prescription, direction, supply and reporting of the following medications, including medications in fixed-dose combinations through Mipres, which are identified by their international common name (INN) in accordance with Resolution 3311 of 2018 or the regulation that modifies, replaces or repeals it,” the circular states.
In total, 19 antiretrovirals are covered by the law, including the well-known dolutegravir, which the government granted a compulsory license to facilitate access to treatment for HIV patients.
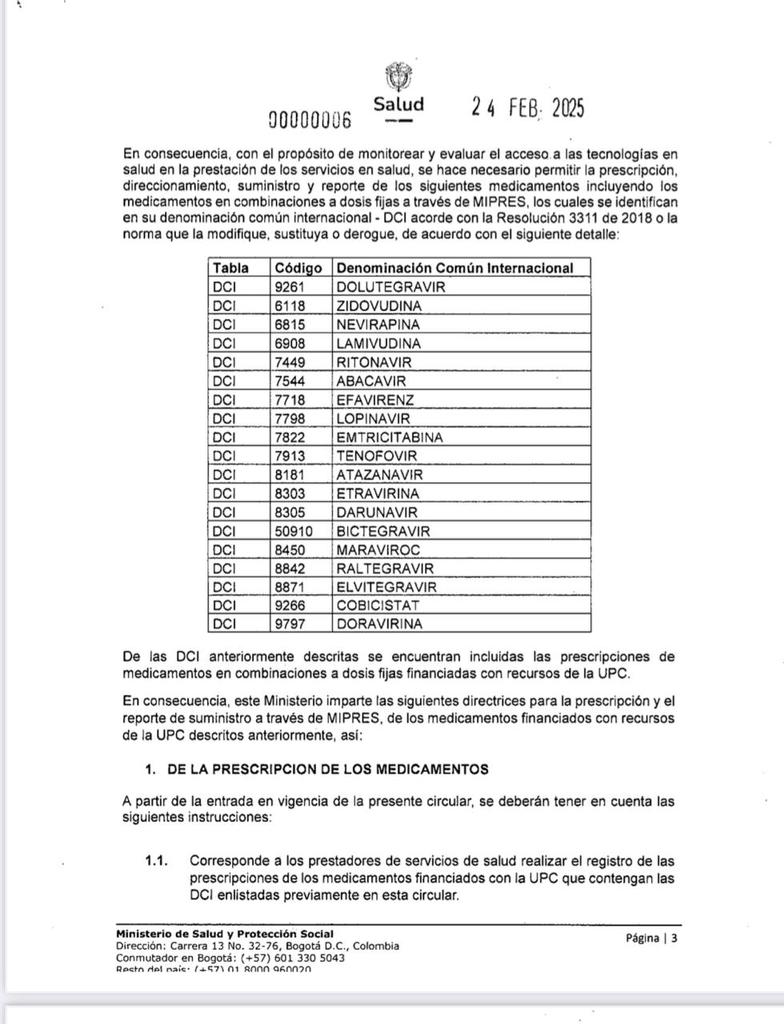
Antiretrovirals that must be prescribed through Mipres. Photo: Ministry of Health
However, the decision of the Ministry of Health has generated concern among the infectious disease specialists' association, who have described this process as a new barrier to access to treatments. "It is unacceptable to increase barriers instead of reducing them! There is an urgent need for unhindered access to medicines, to avoid stigmatization and to speed up care with a comprehensive approach, eliminating administrative barriers," said the Colombian Association of Infectology, which brings together infectious disease specialists, microbiologists, nursing and bacteriology professionals in the country.
According to its director Germán Camacho, it is a “contradiction” that while on the one hand the Government is improving access to the drug by lowering the price of dolutegravir, at the same time it is making access more difficult by adding this new step to obtain it.
Vials of the HIV drug dolutegravir arriving in Colombia. Photo: Ministry of Health
“By asking for it to be done through Mipres, it is an additional administrative step that was not being done and that becomes a barrier for patients, since it is an additional procedure that can generate problems such as the EPS not authorizing it or returning it. Our concern is that this becomes a barrier to access to medicines. The more administrative procedures are requested to justify a formulation that is within the Health Benefits Plan, the more barriers there may be to its delivery. That is our position and our concern,” added Camacho.
Environment and Health Journalist
eltiempo






